Hilleman Laboratories, a not-for-profit venture, is focused on developing innovative technologies and vaccines in areas of unmet need in low- and middle-income countries

India accounts for approximately one-fourth of global deaths from vaccine preventable diseases (VPD). According to the 2015 Global Burden of Disease Report, diarrheal diseases were collectively responsible for 1.3 million deaths across all age groups around the world. Out of these, 500,000 deaths occur in children less than five years of age, a majority of them in India. Diarrhoeal and enteric diseases continue to be a prominently fatal disease in India, despite the availability of simple treatment solutions.
New Delhi-based Hilleman Laboratories is a not-for profit organization that acts as a catalyst in bridging the gap between academic research and product development by targeting novel vaccines and increasing the efficiency of existing vaccines. Hilleman Labs is working with the world’s leading scientists, public & private sector partners, procurement and funding organizations to develop affordable vaccines. Founded by MSD and Wellcome Trust in 2009, Hilleman Labs is the only center of its kind in South Asia, strategically located in India to forge partnerships and strengthen public health in the developing world.
“Typically, vaccines are developed and manufactured in first world countries, aimed mainly at the high-income populations and for local common diseases,” points out Dr Davinder Gill, CEO, Hilleman Laboratories. Therefore, diseases typical of third world countries often get neglected. Moreover, first world vaccines tend to be unaffordable in developing countries. Hilleman Laboratories aims to address these issues by focusing on developing innovative, indigenous vaccines based on the concept to proof-of-concept approach.
As an example, Hilleman Laboratories received a six million-krona Indo-Swedish grant in 2017 for developing an improved, affordable oral cholera vaccine. The project is being jointly funded by the Department of Biotechnology (DBT), Government of India, and Vinnova, the Swedish Governmental Agency for Innovation Systems.
In early 2018, Hilleman Labs partnered with the Future Vaccine Manufacturing Hub led by Imperial College, London, established to increase immunization coverage across the globe and improve the response to disease outbreaks through the rapid and cost-effective deployment of vaccines. The organization also received a grant of £10 million from UK Department for Health in the same year for research and development of high-quality vaccines.
Licensing the technology
Given the need to bridge the innovation gap, Hilleman Laboratories decided to keep its focus on R&D and not enter the manufacturing and market side of the business. “We will license our clinically proven vaccines rather than making capital investment directly in manufacturing and invest the funds and revenues we generate for further research,” explains Dr Gill.
Countdown Begins
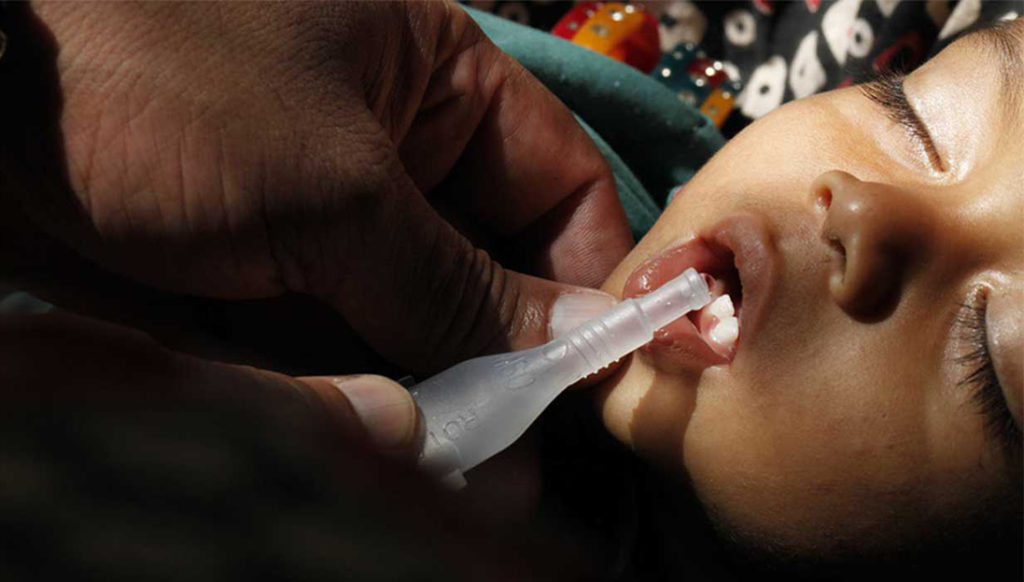
In collaboration with Gotovax AB (a University of Gothenburg spin-off biopharmaceutical company), Hilleman Laboratories aims to deliver a high impact oral cholera vaccine at a significantly more affordable price than the one currently available in the market.
Cholera, an endemic disease in over 50 countries with estimated mortality of 100,000-120,000 deaths and a morbidity of 3.8-4.4 million annually, is another area of focus. Hilleman Labs expects its oral cholera vaccine to be available in the public health system in next two to three years. “The vaccine will be easy-to-administer with cross protection against ETEC diarrhoea and enhanced with a longer shelf life, most suited for geographies with the highest cholera burden such as Africa and South Asia, explains Dr Gill.
Hilleman Labs has previously announced the signing of an MoU with International Centre for Diarrhoeal Disease Research, and Incepta Vaccine Ltd, based in Bangladesh for further development of the vaccine.
Shigellosis, another leading cause of death and disability in children worldwide, especially in low income countries of Africa and South Asia, results in severe diarrhoea. It is responsible for 164,000 deaths, mostly in Africa, South Asia and India. To date, there are no approved vaccines for prevention of disease causes by Shigella. Hilleman Labs is developing a first-of-its-kind vaccine for Shigella infection in collaboration with National Institute of Cholera and Enteric Diseases (NICED), Kolkata, and the Indian Council of Medical Research (ICMR).
If trials succeed, Dr Gill said that the vaccine could take five to seven years to hit the market and would be affordable.
Apart from these, other areas of research and development include the development of ETEC Vaccine and low-cost combination vaccine for treatment of invasive Meningococcal disease.

Innovations at heart
In addition to developing the vaccines, the organization is also undertaking innovations in vaccine delivery and administration. Typically, vaccine formulations perish when taken out of the cold chain and kept in room temperatures. Hilleman Laboratories has developed a thermostable vaccine technology which increases the shelf life of vaccines for four to six months, thereby making it suitable for the climatic conditions of the different parts of the country.
“Another
important innovation that we are working on is simple-to-administer, painless
micro-array patches which do not require intervention of trained nurses or
doctors. The micro-array patch approach helps use a much smaller dose and in
turn maximize the amount of vaccine that is available. The technological
innovations are aimed at making the vaccines easy to use and reduce wastage,”
says Dr Gill. This makes it agile, and along with affordability, has given it
an edge over competition both at the local and global level.
Sector Overview
As a country, we have made significant progress in public health with eliminating maternal and tetanus (MNTE), increasing the full immunization coverage under Mission Indradhanush and reducing under-five deaths due to diarrhoea, pulmonary infections and malnutrition. Last year, we also saw the roll out of Ayushman Bharat initiative which aims to make healthcare affordable and accessible for all.
Today there is a greater political will and accountability towards healthcare in India. There is a growing need for innovations in the field of public health to empower communities with better diagnostics, point of care, and life-saving vaccines.
However, the expansion of vaccine research and development has not happened yet and may take some time. To speed it up, the government should leverage awareness, ease regulations and incentivise innovations.
